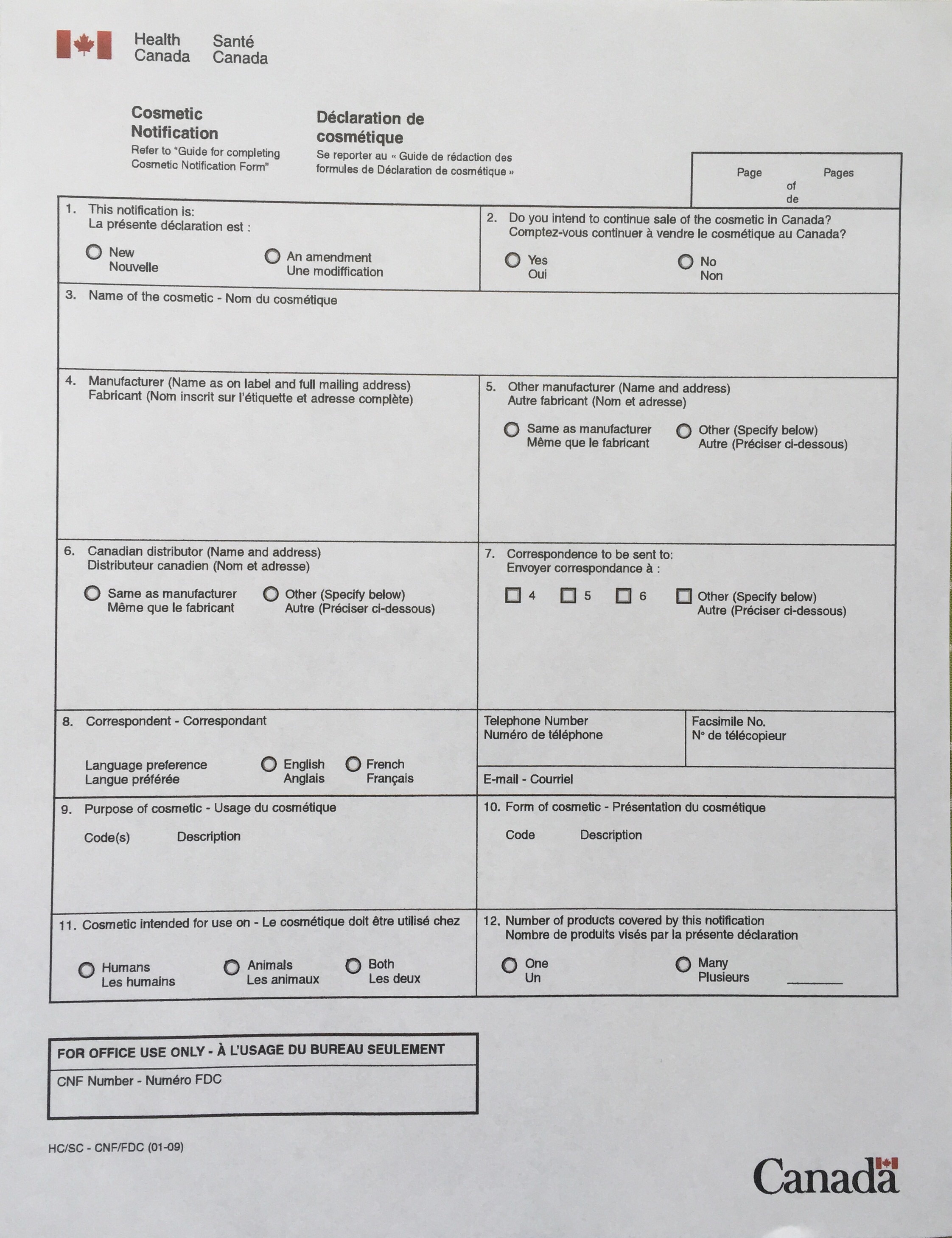
Upcoming Changes to the Cosmetic Notification Form
Straight from the Cosmetics Mailing list:
We are writing to inform you about upcoming changes to the Cosmetic Notification form that will be implemented as a result of the coming into force of the Regulations Amending Certain Regulations Concerning the Disclosure of Cosmetic Ingredients: SOR/2024-63 (amended Regulations).
Some key highlights of the changes are listed below:
Section 2 – Product
| New field: “Type of product”: As per paragraph 30(2)(c) of the Cosmetic Regulations, notifiers will be required to indicate whether the cosmetic is considered leave-on or rinse-off.The field “Area of Application” must be filled out. You will be required to indicate the area of the body where the product would be applied. |
Section 3 – Notifier
| The field “Notifier”: The notifier can be the manufacturer, an importer or a person filling out the notification on their behalf. All correspondence regarding the notification will be sent to the notifier. |
Section 4 – Manufacturing and Label Contact
| Section 4 will be renamed to “Manufacturing and Label Contact”. Notifiers will be required to create a contact-Type for both the “Manufacturer” (as defined in the Regulations) and the “Label Contact”.You will have to enter the contact information for the following (as applicable):the manufacturer (as defined in the Regulations);the importer; andthe person who manufactured or formulated the cosmetic.As per the amended Cosmetic Regulations, a “manufacturer” means the:Canadian manufacturer of cosmetics who sells cosmetics under their own or a trademark, design, trade name, or other name they own or control;Responsible person in Canada acting on behalf of a manufacturer who is not in Canada; orIf no one in Canada meets the definition of the “manufacturer”, the importer or third-party manufacturer in Canada is considered the manufacturer.You can also add contacts for any additional manufacturers, importers, third parties and agents authorized to act on behalf of the notifier in this section.An address in Canada will be required in Section 4 for the form to be successfully submitted. NOTE: Please be informed that this requirement will be implemented at a later date. A date has not been set at this time; however, a message will be sent via the Cosmetics mailing list to advise stakeholders before this functionality update will occur.Use the contact-type “Label contact” to provide the contact information as it appears on the label. This can be either a telephone number, email address, website address, postal address or any other information where consumers can direct their questions about the cosmetic. |
Section 5 – Product Ingredients
| Using the International Nomenclature of Cosmetic Ingredients (INCI) will be mandatory except when it is unavailable, in which case chemical names must be indicated. Users will be able to initiate a search by typing the first few letters of the ingredient, select “Begins With” or “Contains”, click on “Find INCI Name” to see options and then select the appropriate INCI name from the ingredient that is present in the notified product.New Field “Exact Concentration” will be added to allow notifiers to indicate the exact concentration of each ingredient in their product.The concentration ranges will be revised to reflect the updated ranges from the amended Regulations.You can select either the concentration range or exact concentration box for each ingredient.Upon validation, if the status of one or more ingredient is described as “restricted”, you will be required to submit the copy of the label text in Section 6.The document type to be selected must be “Label – Text” or “Label – Marketplace”.As per paragraph 30(1)(b) of the Cosmetic Regulations, if a cosmetic presents an avoidable hazards and requires direction for safe use as per section 22 to 24, the labels and inserts must be provided. This is an updated functionality to help facilitate the processing of cosmetic notifications. |
Section 8 – Submit (previously Section 7)
| New Field “Declaration”: all notifiers will be required to check the box with the following Declaration:I hereby declare that all the information I am submitting is, to the best of my knowledge, true, accurate, current, and complete.I understand that providing false or incomplete information may result in the cosmetic notification form not being processed, which can result in compliance and enforcement actions.New Field “Signature”: All notifiers will be required to input their full name in the signature field. The name provided must match exactly with the one listed in the Contact Person field under the Notifier Section. |
These updates are expected to be implemented on October 9, 2024. Thank you for your attention and continued support. If you have any questions or need further information, please do not hesitate to contact us at cosmetics@hc-sc.gc.ca.
Best regards,
Consumer Product Safety Program
Consumer and Hazardous Products Safety Directorate
Healthy Environments and Consumer Safety Branch
Health Canada

One Comment
Millie G
Hello,
I am assisting my friend with her want of creating and selling bath bombs in BC, Canada. She wants to sell at local markets. I read that you only need to submit a CNF within 10 days after the first sale. So is she okay with not doing it until after her first market? And will she still need to have ingredient list labels for her first market?
Also I have been reading that mica dyes are the only approved dyes and other dyes need batch certification, but realized that’s for USA’s FDA. Can we use any dye in Canada, or do we have similar laws?
Much appreciated,
Millie