Upcoming Changes to the Cosmetic Notification Form
Straight from the Cosmetics Mailing list: We are writing to inform you about upcoming changes to the Cosmetic Notification form that will be implemented as a result of the coming into force of the Regulations Amending Certain Regulations Concerning the Disclosure of Cosmetic Ingredients: SOR/2024-63 (amended Regulations). Some key highlights of the changes are listed below: Section 2 – Product New field: “Type of product”: As per paragraph 30(2)(c) of the Cosmetic Regulations, notifiers will be required to indicate whether the cosmetic is considered leave-on or rinse-off.The field “Area of…
Health Canada proposed changes to Hotlist
(le français suit…) Cosmetics Program Consumer Product Safety Directorate Health Canada Ottawa, Canada June 29, 2017 Subject: Proposed changes to the Cosmetic Ingredient Hotlist Dear Stakeholder: The purpose of this notice is to inform you of substances that Health Canada is currently reviewing and that may be added to the Cosmetic Ingredient Hotlist (Hotlist). We invite you to provide any safety information or other considerations about these substances to Health Canada by August 11, 2017. Please Note: The information in this notice is not currently…
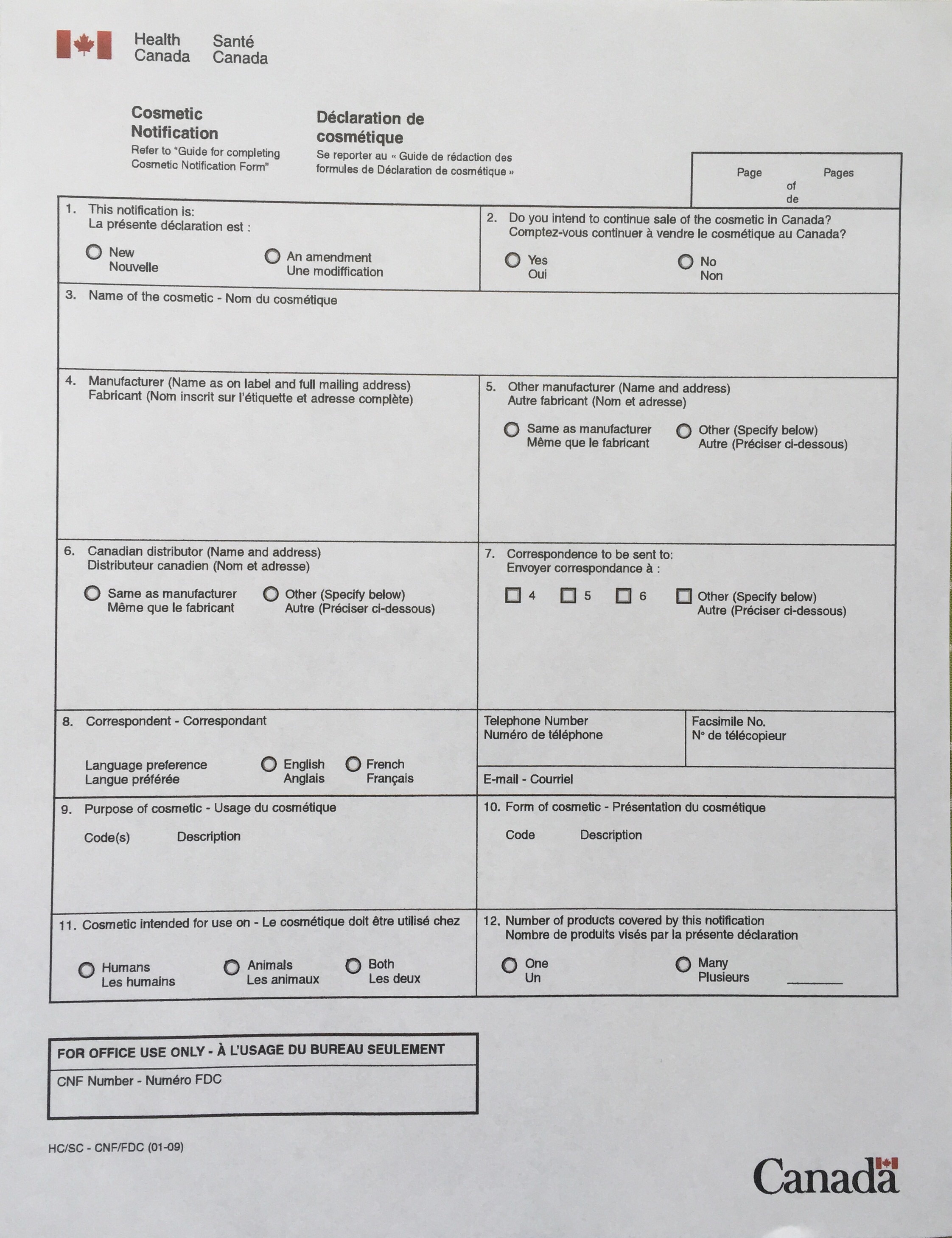
New format to CNF (Cosmetic Notification Form) coming Summer 2016
Health Canada’s Cosmetics Unit is (finally) changing their CNF format from the pdf forms we all know and love-to-avoid, to an hopefully much more user-friendly HTML (website) form. Below is the original newsletter email. Because this information is not found on their website anywhere, before we posted this, we did some fact checking and asked our Cosmetics division contact to confirm the change, and that email is below that! What do you think of this change? From: Cosmaliste <cosmaliste@HC-SC.GC.CA> Date: April 22, 2016 at 2:50:23 PM…
Cosmetic Notification Forms (CNF) – Part Two
Wow, this month is flying by! I apologize for the delay in getting Part Two of this series on CNF published, especially since I know it is on the mind of so many of you these days. In the first part of this series we talked about what a CNF is, and when you would need to file one. If you missed it, you can find it here. Today I’m going to talk a little bit about completing the actual filing process. A blog is not…
Cosmetic Notification Form (CNF) : Part One.
If you are new to the handcrafted bath and body industry in Canada, you may be wondering what all the fuss is about the Cosmetic Notification Form (CNF). I started to write a short post about CNFs, which quickly turned into an encyclopedia, so I have turned it into a two or three part series. Up first, what is a CNF and why do I need one? Health Canada requires that anyone who sells a cosmetic product in Canada must file a CNF with them within…